
China’s biopharma sector strikes $30b in oncology licensing deals
Antibody-drug conjugates dominated licensing activity in 2024.
China’s biopharmaceutical sector saw a sharp rise in oncology drug licensing deals in 2024, especially for monoclonal antibodies (mAbs) and antibody-drug conjugates (ADCs), with a combined deal value of $30b.
“The mAbs and ADCs licensed from Chinese biopharma accounted for 89% of all molecule types, with the total deal value being three times that of similar deals licensed out from the US,” according to a GlobalData report.
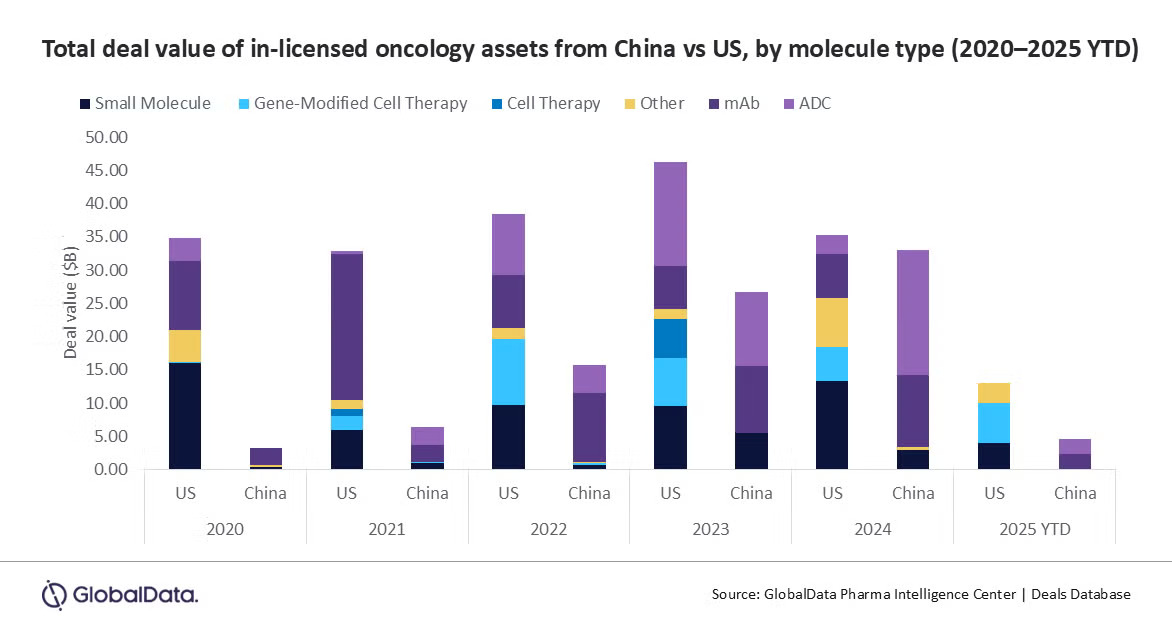
In 2024, ADCs dominated oncology licensing activity in China, accounting for 56% of the total deal value at $19b, followed by mAbs at 33%and small molecules at 9% .
The development is attributed to government policies prioritising innovation, with significant reforms in development processes and regulatory reviews accelerating drug approvals.
However, persistent uncertainties and high tariffs may hinder economic growth and cross-border licensing.
“Temporary tariff reductions provide short-term relief, however shifting policies and potential new restrictions may disrupt the existing agreements and deter future partnerships,” said Ophelia Chan, Senior Business Fundamentals Analyst at GlobalData.

















 Advertise
Advertise









